Basic Guide to Process Validation in Pharma

Back in the 1960s, pharmaceutical products have only been tested after production. If the final product met the standards, it would be cleared for patient use. This approach remained unchanged until the 1970s when a series of incidents shook the industry. One of the most tragic was the thalidomide disaster, in which pregnant women prescribed the drug to treat morning sickness suffered severe birth defects. This made drug developers realize that flaws in the manufacturing process could go unnoticed. By the late 1970s, the concept of process validation was introduced.
How did the validation process change the industry and the lives of millions of people since then, and what does it look like now, in the age of advanced digital tech? This article will discuss pharmaceutical validation, its benefits, types, and basic recommendations. Keep on reading!
Understanding Process Validation in the Pharma Industry
History shows that just meeting final product standards is not enough. Gathering data throughout the production process is essential to ensure safe, high-quality pharmaceuticals.
Pharmaceutical validation involves evaluating each manufacturing stage to ensure that predefined standards are consistently met. It also requires documenting the entire process, from raw material sourcing to product launch.
Why Pharmaceutical Process Validation Is Important
Let’s list the key benefits pharmaceutical process validation offers to drug developers:
It ensures patient safety
Pharmaceutical validation enables pharmaceutical companies to ensure drug safety. Errors in the manufacturing process can lead to defects in the drug, potentially putting patients’ health at risk. Assessments throughout the production lifecycle help guarantee that a drug is safe for consumption.
It promotes consistent product quality
Inconsistent product quality can have serious consequences for patients. Even small deviations in the production process can reduce product efficacy and compromise patient safety. By adhering to critical process parameters (CPPs), pharmaceutical companies can avoid legal and financial risks while building trust with patients in the safety and reliability of their products.
It cuts down costs
Cost reduction is a key commercial benefit for any business. Regular assessments of manufacturing stages not only ensure drug quality but also help reduce resource spending. By optimizing each process, pharmaceutical businesses can minimize waste throughout the production lifecycle.
It ensures compliance
The Food and Drug Administration (FDA) in the U.S. and the European Medicines Agency (EMA) strictly regulate the manufacturing process to ensure that end users receive effective therapies without health risks. Process validation helps companies maintain transparency with regulatory bodies and comply with current laws.
It supports customer loyalty
The key to winning loyal customers is delivering quality products consistently. Research shows that a staggering 73% of customers will abandon a brand after just one negative experience. This percentage is even higher in the pharmaceutical industry, where human life and health are on the line. Process validation helps companies continually meet quality standards, earning the trust of both healthcare providers (HCPs) and patients.
Types of Process Validation
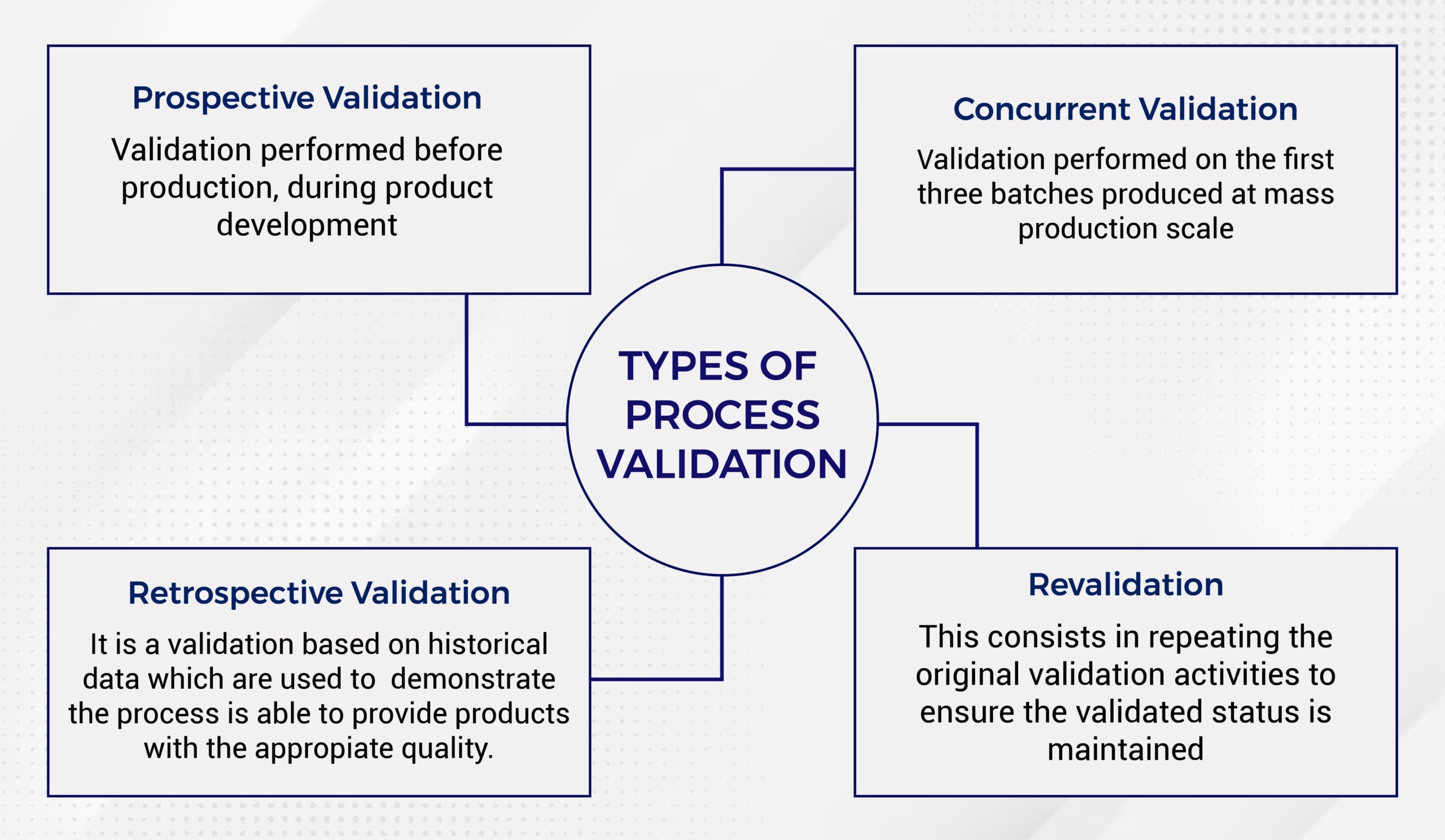
Source: Operon Strategist
Four types of process validation are:
Prospective validation
This type of process validation occurs during the development stage before product marketing to consumers. The primary objective is to ensure that the production design meets all necessary criteria.
Concurrent Validation
Concurrent validation involves gathering real-time data during actual production runs. This type of validation is particularly useful for fast product launches (think the COVID-19 vaccine) when there is no time for prospective validation.
Retrospective Validation
Unlike concurrent validation, retrospective validation relies on historical data from previous production runs. It is often used for well-established processes that consistently demonstrate strong performance over time.
Revalidation
Companies use revalidation when they significantly change raw materials, manufacturing processes, or equipment. The main goal is to ensure that these alterations have not impacted production and that everything continues functioning as expected.
Key Steps of Process Validation
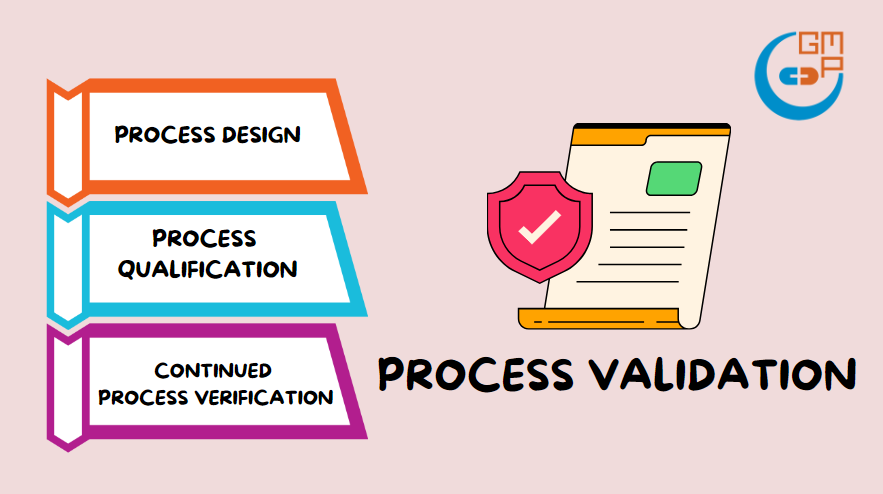
Source: GMP
There are three fundamental steps in process validation:
Process design
The goal of the process design stage is to find the right way to produce the product. Process controls ensure the drug’s safety and effectiveness by monitoring equipment and conducting tests. This is especially critical when intermediates are fully developed, and it is difficult to measure the drug’s properties.
Process design should be based on solid evidence and include thorough documentation. Stakeholders need to record the studies that have helped improve their understanding of the manufacturing processes.
Process qualification
At this stage, the drug developer must confirm whether the process design works effectively for commercial use. It is essential to choose the right utility systems and equipment that meet the design standards. After that, the manufacturer has to make sure everything functions properly.
Process performance qualification (PPQ) involves utilities, the facility, equipment, and trained staff. The FDA suggests using measurable data to monitor performance accurately.
Teams also need clear protocols that cover data collection, production conditions, the sampling plan, and any necessary tests. The PPQ protocol should only be implemented after all major departments have signed off on it.
Process verification
Continued process verification aims to ensure the process stays approved during commercial production. For this reason, it is important to continuously collect and analyze data on drug quality to spot any changes or issues that need to be addressed.
The FDA recommends ongoing sampling and performance tracking until enough data is gathered. It is also crucial for stakeholders to maintain the utilities, equipment, and facilities.
Key Trends in Process Validation
Tech disruptions have impacted every part of the pharmaceutical industry, and process validation is no exception. Here are some of the key trends we are seeing right now:
Process analytical technologies
As the name implies, process analytical technologies (PAT) use analytical tools to monitor, manage, and control drug production on the spot. Unlike the traditional approach that centers on controlling the quality of each batch, these technologies allow for dynamic management, helping to detect and correct errors on the spot.
Let’s break down the key benefits so you understand whether this type of solution is right for you:
Early error identification
Tools like in-line sensors, chromatography, and spectroscopy empower life sciences teams to spot defects in real time. Comparing the instant data to pre-defined standards allows companies to quickly detect deviations, thereby reducing waste and improving drug manufacturing efficiency.
Fact-based decision-making
PAT provides a wealth of up-to-date data, allowing stakeholders to make strategic decisions instead of relying on blind guesses. This speeds up decision-making, enabling brands to catch quality issues early and launch products faster than their competitors.
Automation
PAT solutions can be merged with process control systems. When an error is detected, the system can automatically correct it. This level of automation helps keep the manufacturing process consistently error-free.
Cloud-based quality management solutions
Updating traditional quality management solutions is not easy. Pharma teams often worry about the added costs and potential production delays. On the other hand, avoiding updates makes it harder to stay afloat and competitive.
Cloud solutions are becoming a popular trend for process validation, helping companies meet industry standards with less effort and expense. Its major gains include:
Incremental updates
Updates are handled gradually, causing minimal disruption, and there is often a rollback feature that allows users to undo changes with little downtime.
No need for infrastructure
Another advantage is that cloud quality management systems do not require additional infrastructure. This cuts costs and gives you greater freedom to scale at your own pace.
Team of seasoned experts
Many cloud providers offer validation experts who help life sciences brands improve their validation processes. Their goal is to reduce manual work so teams can narrow-focus their attention on core business operations.
What Are the Challenges and Considerations to Check Out?
As you have probably guessed, pharmaceutical validation is complex and full of roadblocks and potential pitfalls. Let’s take a closer look so we can be better prepared for them:
Identifying key process parameters
Quality teams must know which attributes to monitor to ensure the manufacturing process runs smoothly. That is why many organizations turn to data analytics to pinpoint the parameters that impact production the most.
Cross-functional collaboration is often necessary. Companies can more easily identify the right attributes and parameters by bringing together teams from production, R&D, and quality assurance.
Adapting to new technologies
Given the pharmaceutical industry’s high-risk nature, it is no surprise that many companies are cautious about digitalization. Pharma brands often take their time adopting new solutions, especially in production processes.
In this case, the best strategy is to eat a giant elephant with a teaspoon. It is important not to implement every solution under the sun across the entire validation process. Start small – test demo versions, set clear performance indicators, gather employee feedback, and evaluate the results before moving forward.
Documenting all manufacturing stages
Very few people enjoy the painstaking work of documenting a lengthy process in detail. It demands patience, attention to detail, and the readiness to make necessary edits along the way.
Start by setting clear goals for organizing your documents and think about how you will store and manage records. You should decide what data to collect and how to categorize it. A solid data management system will help you avoid data silos, duplicate documents, and incorrect data tagging.
It is worth noting that not backing up regularly is one of the biggest common mistakes. Setting up automatic backups can save your team from the nightmare of recreating documents from the ground up.
Ensuring Smooth Validation in the Pharmaceutical Industry
Process validation enables pharmaceutical companies to ensure that every step of the manufacturing process contributes to producing effective and safe drugs. Adhering to all regulations and standards is crucial for maintaining consistent product quality.
Process validation has not escaped the wave of digitalization that has touched every part of the industry. Recently, real-time analytics and cloud solutions have gained popularity – and for good reason. They facilitate continuous quality control for each batch and keep the quality management tools up and running.
If you are considering moving to the cloud, building a quality management system, or developing an analytical tool, the Viseven team has the expertise to meet your needs. Recently, we celebrated our 15th anniversary – 15 years of creating an average of 35 innovative healthcare solutions each year. More than 90 seasoned tech experts are behind these impressive numbers.
Before you go, here is another statistic: 80% of our clients come from referrals. If you want to become one of our happy customers, Contact Us and let’s start a conversation.



